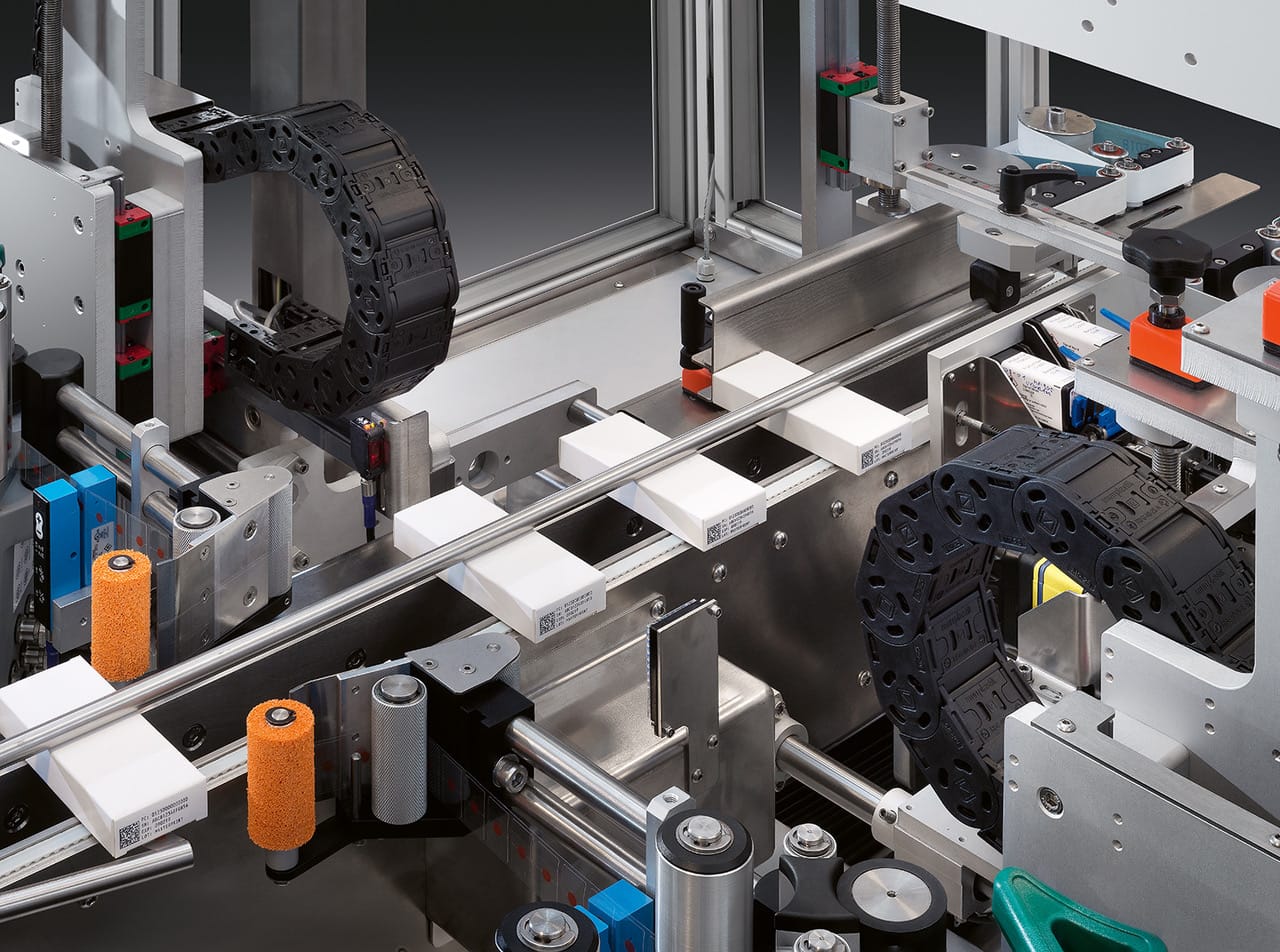
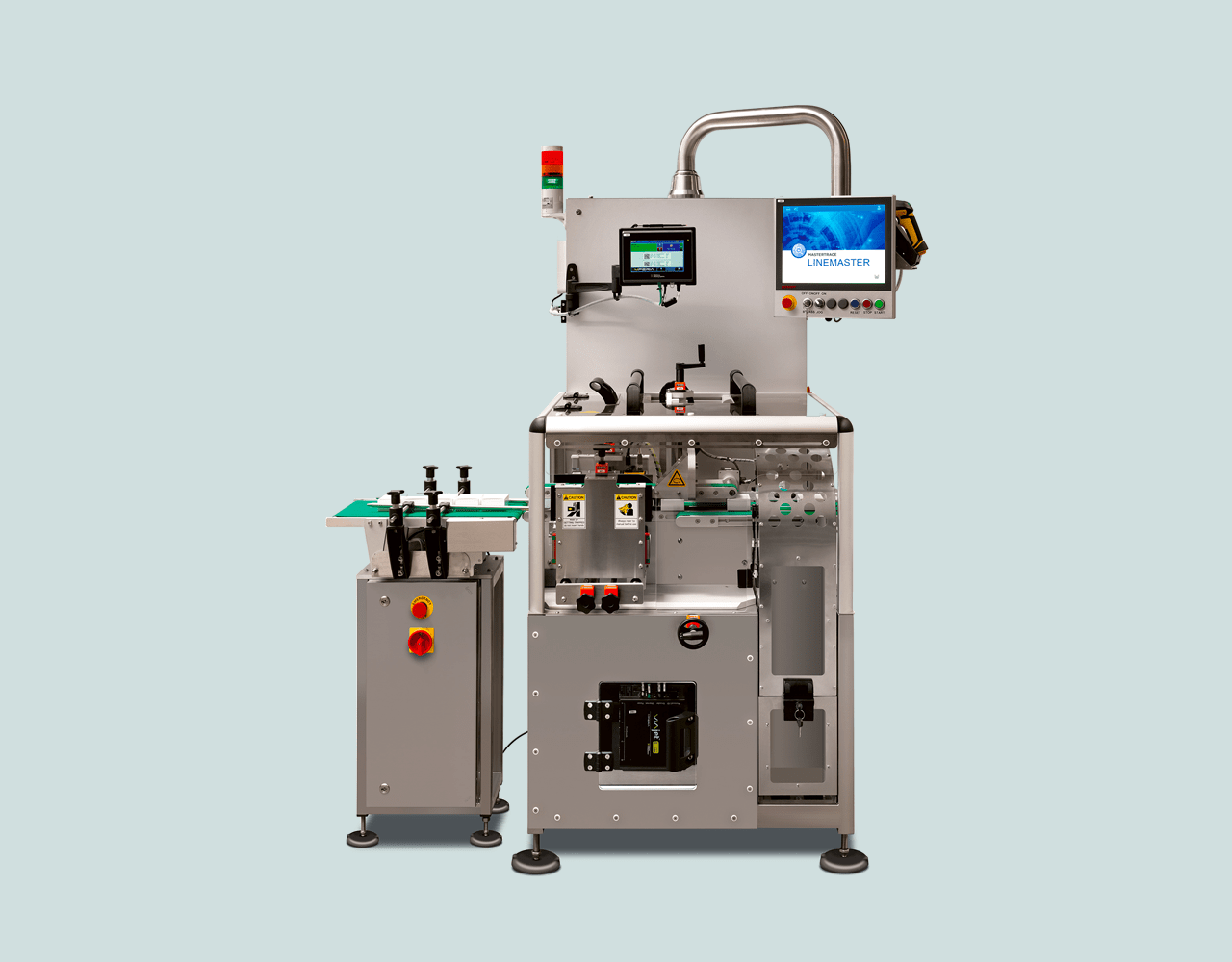
Our serialisation station is a complete solution for the labelling and aggregation of pharmaceutical products. The hardware and software system covers the entire process from L1 to L4. Flexible configuration options allow the equipment to be easily integrated into existing production processes, making the transition to the new structure quick and seamless.
PharmaJET P&C TE Aero performs the automated serialisation of pharmaceutical products in the following steps:
1, Dosage
The equipment first performs the segmentation and dosage. With manual dispensing, the machine checks the positioning of the boxes to ensure that each product is in the correct position for marking and labelling. Optionally, a control scale can also be integrated in the system.
2, Pre-marking product control
Before marking, the system inspects the loaded boxes for several requirements: incorrect box orientation, open box loops, incorrect orientation or incorrect weight.
3, Serialisation
It is possible to print product information according to EU standards with thermal inkjet or laser markers. Next, the bottom and top tamper-evident labels are applied to the boxes. Products are transported between each station by a conveyor belt with guide vanes. The serialisation system can be integrated with any L3-L4 software service.
4, Marking and labeling control
Once the labels and inscriptions have been applied to the medicine boxes, a total of three built-in Track and Trace cameras monitor whether the serialisation has been done correctly and check for the presence of the labels. If the system detects an anomaly, it uses an air blower to sort out the defective boxes into a separate bin.
5, Shipment management
The last step is the deaggregation and reaggregation of the returned items, which can be done manually, semi-automatically or automatically.