Pharmaceutical
Serialisation

AN EFFECTIVE TOOL FOR ELIMINATING COUNTERFEIT PRODUCTS
Why is it necessary?
Traceability standards have been introduced by various international and national trade bodies to detect counterfeits and to ensure that products can always be traced back to their manufacturer.
Benefits
- Transparent supply chain
- Increases professional and consumer confidence in brand products
- Simple and efficient product recall
- Monitoring of the consumption rate
- Marking tasks can be seamlessly integrated into the production workflow
- Solution from L1 to L4 (software and hardware together)
Why is serialisation important in the pharmaceutical industry?
Counterfeit products, which are continuously appearing on the market and usually imitate the appearance of genuine brands but fall far short of industry quality standards, are a serious risk to the pharmaceutical industry worldwide. If they contain any active substances at all, they do not contain the required amount, which in some cases can make them life-threatening for their end users.
How medicines are identified varies from country to country, but actually the same information should always be included: a unique serial number, identification code, batch number and expiry date. In Hungary, the OGYÉI [National Institute of Pharmacy and Nutrition] or EU authorisation number and information in Braille are mandatory on consumer packaging.
Serialisation in the pharmaceutical industry is also important for manufacturers, as it allows them to prove the authenticity of their products without fear of regulatory penalties for counterfeits. However, it is also important that serialisation does not reduce the efficiency of production processes. For this reason, Industry 4.0 concepts are also playing an increasing role in the pharmaceutical industry. Automated serialisation stations not only increase productivity, but also minimise the possibility of errors because they require fewer human resources.
INDUSTRIAL APPLICABILITY:
IN THE PHARMACEUTICAL INDUSTRY, PRODUCTS CAN BE MARKED AT THREE LEVELS

Although serialisation was originally developed specifically for the pharmaceutical and chemical industries, the methods that have been proven in these industries can also be useful in many other sectors.
There are areas where serialisation is not required by specific legislation, but by strict health requirements. Typical industries include:
#FOOD
#AGROCHEMISTRY
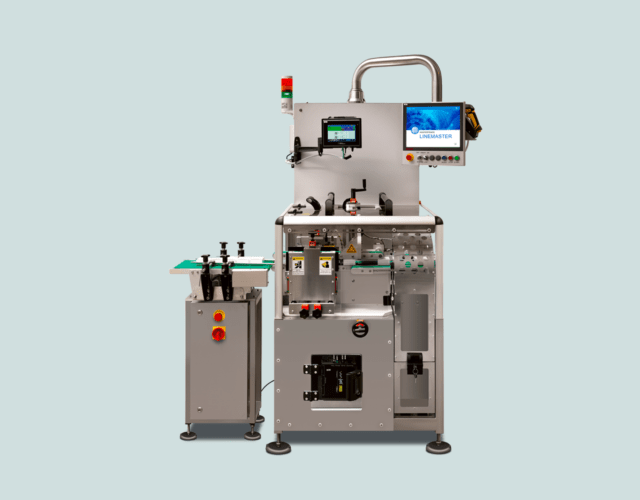
Masterprint Pharmaceutical Serialisation Stations
Thanks to technological innovations, the serialisation of medicines has become a streamlined process that can be flexibly customised. With centralised print control and monitoring systems for product tracking, we can coordinate the hardware and software to develop the most ideal marking method.
With our complete systems, we can serialise medicines in full compliance with Hungarian and EU regulations, from the moment the raw materials arrive at the factory until the products are marketed. Our serialisation stations and print control software modules can be integrated into even the fastest production lines.